
However, these elements are reactive enough that they do not exist in their elemental forms in nature, but are present as compounds. Alkali metals readily lose electrons, making them count among the most reactive elements on earth. The need to remove two electrons in order for the material to react means more energy is needed for electron removal. o Lithium sodium and potassium metal are produced by electrolysis of the molten chlorides. The Group 2 elements tend to be less reactive than their Group 1 counterparts. The higher ionization energy makes the alkaline earth metals less reactive than the alkali metals however, they are still very reactive elements.

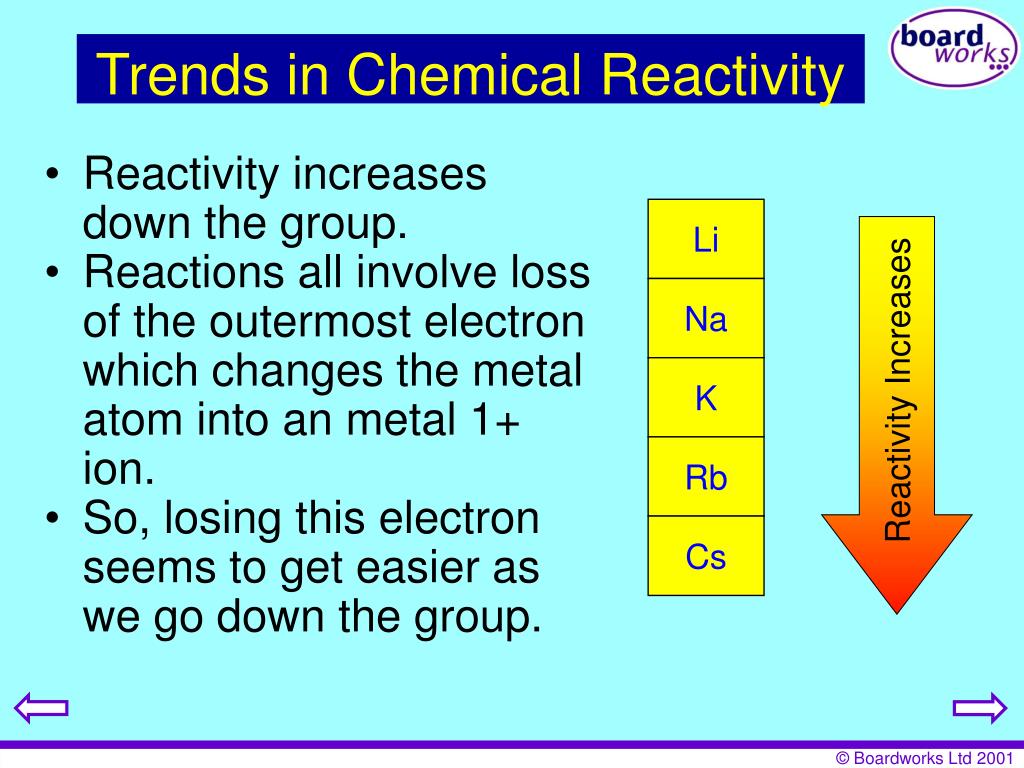
However, radium is a radioactive element and is generally under the category of radioisotopes in addition to being an alkaline earth metal, because it is not a stable element. Radium (atomic number 88) has similar properties to barium and is also in the Group 2 category. The reaction of alkali metals with water is represented by the following equation: 2 M (s or l) + 2 H2 O (l) -> 2 M (OH)2(aq) + H 2(g) Where M is the alkali metal. Alkali and alkaline earth metals can serve both as promoters for methanation catalysts and as adsorbent phases upon the combined capture and methanation of CO 2. Beryllium does not react with water, magnesium reacts with steam. Beryllium is sufficiently hard to scratch glass, but barium is only slightly harder than lead. They have a gray-white lustre when freshly cut but tarnish readily in air, particularly the heavier members of the group. This is because, as explained previously, it is much easier to remove an outer shell. The alkaline-earth elements are highly metallic and are good conductors of electricity. \) (Credit: Ingmar Runge Source: (opens in new window) License: Public Domain) The reactivity of the alkaline earth metal increases as you move down the column. The reactivity of the group 2 elements increase as you go down the group.


 0 kommentar(er)
0 kommentar(er)
